|
 |
||||||||
|
|
||||||||
|
239 Commerce Street w P.O. Box 358 w Glastonbury, CT 06033-0358 w Phone: 860-659-4330 w Fax: 860-659-9561
Custom Hot Dip Galvanizing Protecting Steel for Generations
Please feel free to contact us by phone, fax or email:
Why Why Galvanize? What is corrosion? Corrosion is the reaction between a material and its environment that produces a deterioration of the material and alters its mechanical properties. The actual corrosion process that takes place on a piece of bare mild steel is very complex due to factors such as variations in the composition/structure of the steel, presence of impurities due to the higher instance of recycled steel, uneven internal stress, or exposure to a non-uniform environment. It is very easy for microscopic areas of the exposed metal to become relatively anodic or cathodic. A large number of such areas can develop in a small section of the exposed metal. Further, it is highly possible that several different types of galvanic corrosion cells are present in the same small area of the actively corroding piece of steel. As the corrosion process progresses, the electrolyte may change due to materials dissolving in or precipitating from the solution. Additionally, corrosion products might tend to build up on certain areas of the metal. These corrosion products do not occupy the same position in the given galvanic series as the metallic component of their constituent element. As time goes by, there may be a change in the location of relatively cathodic or anodic areas and previously uncorroded areas of the metal are attacked and corrode. This eventually will result in uniform corrosion of the area. The rate at which metals corrode is controlled by factors such as electrical potential and resistance between anodic and cathodic areas, pH of the electrolyte, temperature and humidity. Go to top
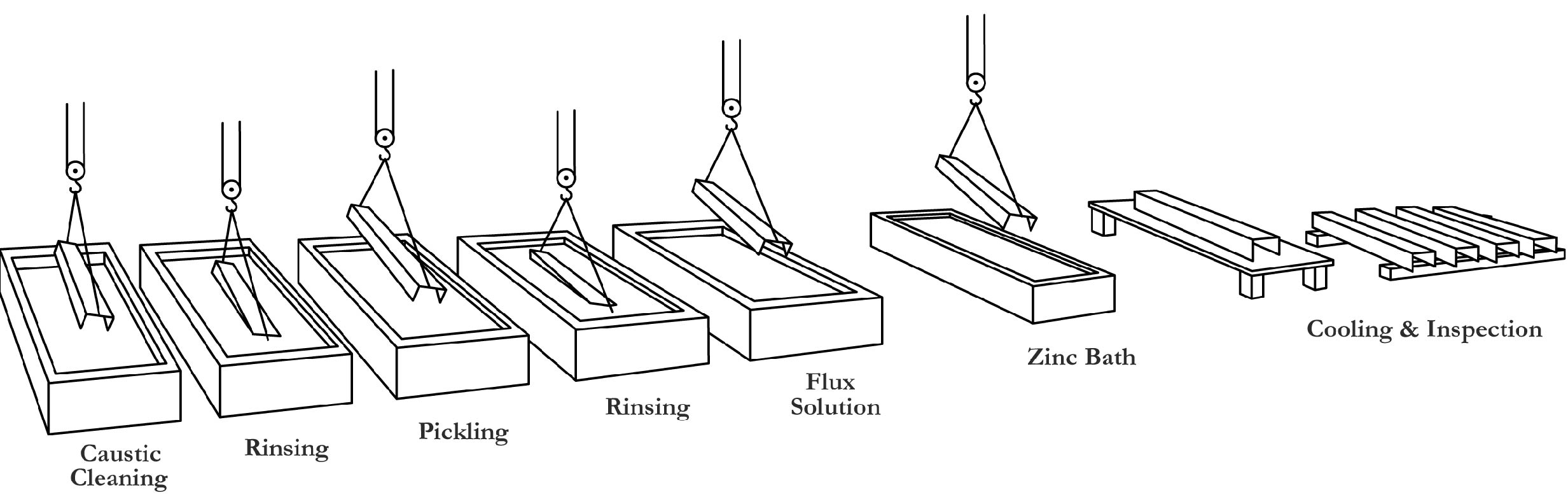
How do you protect iron and steel from corrosion? Barrier protection is perhaps the oldest and most widely used method of corrosion protection. It acts by isolating the metal from the electrolytes in the environment. Two important properties of barrier protection are adhesion to the base metal and abrasion resistance. Cathodic protection is an equally important method for preventing corrosion. Cathodic protection requires changing an element of the corrosion circuit, introducing a new corrosion element, and ensuring that the base metal becomes the cathodic element of the circuit. Hot-dip galvanizing provides excellent barrier and cathodic protection. The sacrificial anode method, in which a metal or alloy that is anodic to the metal to be protected is placed in the circuit and becomes the anode. The protected metal becomes the cathode and does not corrode. The anode corrodes, thereby providing the desired sacrificial protection. In nearly all electrolytes encountered in everyday use, zinc is anodic to iron and steel. Thus, the galvanized coating provides cathodic corrosion protection as well as barrier protection. Go to Index Go to top The galvanizing process consists of three basic steps: surface preparation, galvanizing and inspection. Surface Preparation is the most important step in the application of any coating. In most instances where a coating fails before the end of its expected service life, it is due to incorrect or inadequate surface preparation. The surface preparation step in the galvanizing process has its own built-in means of quality control in that zinc simply will not react with a steel surface that is not perfectly clean. Any failures or inadequacies in surface preparation will be immediately apparent when the steel is withdrawn from the molten zinc. Any areas that were not properly prepared will remain uncoated. Immediate corrective action is taken. Surface preparation for galvanizing typically consists of three steps: caustic cleaning, acid pickling and fluxing. Caustic Cleaning – A hot alkali solution often is used to remove organic contaminants such as dirt, paint markings, grease and oil from the metal surface. Epoxies, vinyls, asphalt or welding slag must be removed before galvanizing by grit-blasting, sandblasting or other mechanical means. Pickling – Scale and rust normally are removed from the steel surface by pickling in a dilute solution of hot sulfuric acid or ambient temperature hydrochloric acid. Fluxing – Fluxing is the final surface preparation step in the galvanizing process. Fluxing removes oxides and prevents further oxides from forming on the surface of the metal prior to galvanizing and promotes bonding of the zinc to the steel or iron surface. The method for applying the flux depends upon whether the particular galvanizing plant uses the wet or dry galvanizing process. In the dry galvanizing process, the steel or iron materials are dipped or pre-fluxed in an aqueous solution of zinc ammonium chloride. The material is then thoroughly dried prior to immersion in molten zinc. Go to Index Go to top
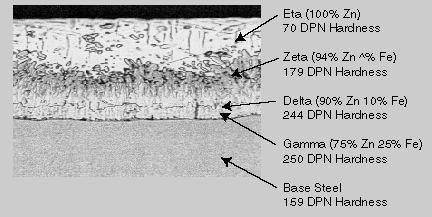
In this step, the material is completely immersed in a bath consisting of a minimum 98% pure molten zinc. The bath chemistry is specified by the American Society for Testing and Materials (ASTM) in Specification B 6. The bath temperature is maintained at about 850 F (454 C). Fabricated items are immersed in the bath long enough to reach bath temperature. The articles are withdrawn slowly from the galvanizing bath and the excess zinc is removed by draining, vibrating and/or centrifuging. The chemical reactions that result in the formation and structure of the galvanized coating continue after the articles are withdrawn from the bath as long as these articles are near the bath temperature. The articles are cooled in either water or ambient air immediately after withdrawal from the bath. The two properties of the hot-dip galvanized coating that are closely scrutinized after galvanizing are coating thickness and coating appearance. A variety of simple physical and laboratory tests may be performed to determine thickness, uniformity, adherence and appearance. Products are galvanized according to long-established, well-accepted and approved standards of the ASTM, the Canadian Standards Association (CSA), and the American Association of State Highway and Transportation Officials (AASHTO). These standards cover everything from the minimum required coating thicknesses for various categories of galvanized items to the composition of the zinc metal used in the process. Go to Index Go to top What is the resulting metallurgical bond? Galvanizing forms a metallurgical bond between the zinc and the underlying steel or iron, creating a barrier that is part of the metal itself. During galvanizing the molten zinc reacts with the surface of the steel or iron article to form a series of zinc/iron alloy layers. The photomicrograph below shows a typical galvanized coating microstructure consisting of three alloy layers and a layer of pure metallic zinc. Moving from the underlying steel surface outward, these are:
Below the name of each layer in the photomicrograph appears its respective hardness, expressed by a Diamond Pyramid Number (DPN). The DPN is a progressive measure of hardness: the higher the number the greater the hardness. Typically, the Gamma, Delta and Zeta layers are harder than the underlying steel. The hardness of these inner layers provides exceptional protection against coating damage through abrasion. The Eta layer is quite ductile, providing the coating with some impact resistance. The galvanized coating is adherent to the underlying steel on the order of several thousand pounds per square inch (psi). Other coatings typically offer adhesion rated at several hundred psi at best. Hardness, ductility and adherence combine to provide the galvanized coating with unmatched protection against damage caused by rough handling during transportation to and/or at the job site, as well in service. The toughness of the galvanized coating is extremely important since barrier protection is dependent upon the integrity of the coating. Other coatings damage easily during shipment or through rough handling on the job site. Experts will argue that all organic forms of barrier protection (such as paint) by their nature are permeable to some degree. Galvanized coatings are impermeable. If the galvanized coating is physically damaged, it will continue to provide cathodic protection to the exposed steel. If individual areas of underlying steel or iron become exposed as much as 1/4", the surrounding zinc will provide these areas with cathodic protection for as long as the coating lasts. The galvanizing process naturally produces coatings that are at least as thick at the corners and edges as the coating on the rest of the article. As coating damage is most likely to occur at the edges, this is where added protection is needed most. Brush- or spray-applied coatings have a natural tendency to thin at the corners and edges. Because the galvanizing process involves total immersion of the material, it is a complete process; all surfaces are coated. Galvanizing provides both outside and inside protection for hollow structures. The galvanizer’s ability to work in any type of weather allows a higher degree of assurance of on-time delivery. Working under these circumstances, galvanizing can be completed quickly and
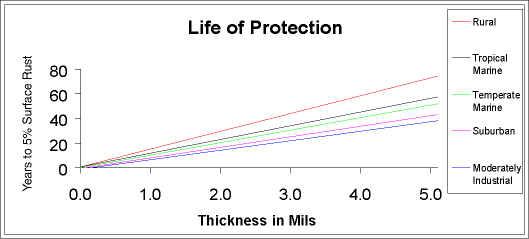
with short lead times. A turnaround time of two or three days for galvanizing is common. What is the expected service life of a galvanized coating under outdoor conditions? The graph below is a plot of the thickness of the galvanized coating against the expected service life of the galvanized coating under outdoor exposure conditions. Most galvanized applications are 4 mils of thickness minimum per surface. The expected service life is defined as the life until 5% of the surface is showing iron oxide (rust). At this stage, it is unlikely that the underlying steel or iron has been weakened or the integrity of the structures protected by the galvanized coating otherwise compromised through corrosion. Graph – “Life of Protection”
Paint and Powder Coating over hot dip Galvanizing (Duplex Coating) For years, protecting steel from corrosion typically involves either the use of hot dip galvanizing or a barrier system such as painting or powder coating. However, more and more corrosion specialists are utilizing both methods of corrosion protection in what is commonly referred to as a duplex system. A duplex system is simply painting or powder coating steel that has been hot dip galvanized. When paint and galvanized steel are used together, the corrosion protection is superior to either protection system used alone. You will have superior corrosion protection and a wide variety of colors for your application. Connecticut Galvanizing exceeds all requirements of ASTM A123 for galvanizing and ASTM 6386-99 for paint. WHY USE A DUPLEX SYSTEM (Paint or Powder Coating over Hot Dip Galvanizing Steel) There is a wide array of reasons for using a duplex system, ranging from financial benefits to safety. Each individual project raises unique reasons why a duplex system should be utilized. Extended Corrosion Resistance – The most obvious, and most important, reason for using a duplex system is the added corrosion protection. No single corrosion protection system can match the corrosion resistance afforded for most applications by painting over hot dip galvanized steel. Aesthetics – Galvanizing has an attractive gray, metallic appearance suitable for a myriad of applications. However, painting the galvanized coating provides an alternative aesthetic appearance. For example, galvanized parts could be painted to match a specific environment, such as at a stadium, a theme park or a natural habitat. Synergistic Effect – It is typical for a duplex system to provide corrosion protection 1.5 to 2.5 times longer than the sum of the lifetimes of zinc and paint used individually. For example, if a galvanized coating is expected to last 40 years and a paint system is expected to last 10 years, galvanizing and paint together should last 75 years with needed maintenance, or 1.5 times the sum of both systems. Economic Benefits – Because duplex systems greatly extend the service life of a product, maintenance costs are significantly decreased. Additionally, a product lasts longer before it must be replaced, thus decreasing the life-cycle cost. The cost of a product, which has been protected by galvanizing and painting, is lower over the entire life of the product than most single system methods of corrosion protection. Safety Marking & Color Coding – The use of duplex systems allows galvanized steel to be painted in order to conform with safety color regulations. Painting over galvanized steel also increases safety in many environments by commonly color coding gas, steam or chemical pipes; identifying hazardous work areas and walkways; and marking high voltage electrical lines. (Paint or powder coating over hot dip galvanizing steel). Ease of Repainting – As the paint film weathers, the zinc in the galvanized coating is present to provide both cathodic and barrier protection until the structure is repainted. The exposed zinc surface then can be repainted with minimal surface preparation. We would like to express our appreciation to The American Galvanizers Association for supplying material and photographs for our web site. Go to Index Go to top |
|
||||||||
|
Member Associations |
|
[Home Page] [Fabricating] [Products] [Contacts] [Galvanizing] |
|
Please contact our Webmaster with questions or comments. |